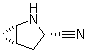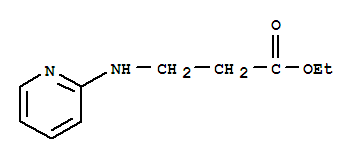Hebei started the small package of traditional Chinese medicine decoction pieces to promote the development of Chinese medicine industry In view of the simple and random packaging of traditional Chinese medicine decoction pieces, which leads to the phenomenon of shoddy filling and doping and falsehood, the Hebei Food and Drug Administration issued a notice requesting the production and operation of Chinese Herbal Pieces from November 1, 2009. Enterprises must use standard material packaging, and the packaging quantity should not exceed 1kg. This indicates that the bulk pieces will be withdrawn from the historical stage in Hebei Province. This will not only help the quality tracking and traceability of Chinese herbal medicines, but also promote the quality of Chinese medicines, promote the development of Chinese medicine industry, and promote Chinese traditional medicine. Notification requirements: 1. Medical institutions and pharmaceutical retail enterprises must purchase decoction pieces with legal packaging and labeling from legal Chinese medicine decoction pieces production and management enterprises, and check the qualifications of salesmen online, and obtain corresponding certificates and bills. 2. The packaging and labeling of traditional Chinese medicine decoction pieces should meet the four requirements. 1. The packaging materials for traditional Chinese medicine decoction pieces must be packaged in plastic materials that are non-toxic, harmless, transparent and meet the edible standards. 2, the amount of Chinese medicine decoction pieces, according to different types of pieces and customer needs, but more than 1Kg. 3. The Chinese medicine decoction pieces purchased from the decoction piece production enterprises shall be marked with the product name, place of origin, specifications, manufacturer's name, drug production license number, product batch number, production date, and the quality qualified mark; implementation approval number management The Chinese medicine decoction must indicate the approval number. 4. The traditional Chinese medicine decoction pieces purchased from the wholesale foods of the pieces should be marked with the name, specification, place of origin, name of the business enterprise, date of manufacture, date of packaging, and the mark of qualified quality. The Chinese medicine decoction pieces purchased from the decoction piece production enterprises and then sub-packaged and wholesaled must also indicate the name of the manufacturer and the batch number of the production. This regulation will be implemented from November 1, 2009. Chinese medicine decoction pieces purchased by pharmaceutical companies and medical institutions may continue to be sold until they are used up, but the time shall not exceed December 1, 2010. |
|
statement:
1. This article is reproduced by the editor of this website. It does not mean that I agree with his opinion and is responsible and verified for his authenticity.
2. If the content, copyright and other issues of the work are related to this article, please contact the website within 30 days, we will deal with it at * time. |
Intermediates of Cladribine, Carvedilol, Lurasidone, olmesartan,
Risedronate Sodium, Atazanavir, Saxagliptin,
Dabigatran,Dapoxetine,Cefixime,Ceftaroline fosamil and etc.
In the short span of time, we have emerged as most promising
pharmaceutical intermediates manufacturers, chemical intermediates and
bulk drug intermediates suppliers. Our consistent supply, quality
products and dedication towards clients have opened up many
international avenues for our growth.
In addition, the company also can follow the customer's product needs custom synthesis services
MAIN API PRODUCTS USP/BP
|
PRODUCT NAME
|
CAS NUMBER
|
SPEVIFICATION
|
|
Azithromycin
|
117772-70-0
|
BEP
|
|
Cefpirome Sulphate sterile
|
84957-29-9
|
USP JP16
|
|
Ceftriaxone Sodium (Sterile)
|
104376-79-6
|
USP31
|
|
Cefotaxime
|
64485-93-4
|
USP30
|
|
Ciprofloxacin HCL
|
85721-33-1
|
USP/BP
|
|
Gentamicin sulphate
|
1405-41-0
|
BP
|
|
Levofloxacin
|
100986-85-4
|
USP27
|
|
Lincomycin Hydrochloride
|
859-18-7
|
EP6.0
|
|
Moxifloxacin Hydrochloride
|
186826-86-8
|
USP31
|
|
Tigecycline
|
220620-09-7
|
USP
|
|
Linezolid
|
165800-03-3
|
EP
|
|
Dexamethasone
|
50-02-2
|
USP/BP/EP
|
|
Methylprednisolone
|
83-43-2
|
USP/BP/EP
|
|
Dexketoprofen trometamol
|
156604-79-4
|
BP2008
|
|
Ibuprofen
|
15687-27-1
|
BP
|
|
Metamizol
|
68-89-3
|
DAB
|
|
Sulindac
|
38194-50-2
|
USP/BP/EP
|
|
Naproxcinod
|
163133-43-5
|
USP28
|
|
Tripelennamine Hydrochloride
|
154-69-8
|
USP28
|
|
Itraconazole
|
84625-61-6
|
USP/BP
|
|
Cytarabine
|
147-94-4
|
USP31
|
|
Leucovorin Calcium
|
1492-18-8
|
USP32
|
|
Valsartan
|
137862-53-4
|
USP30
|
|
Telmisartan
|
144701-48-4
|
USP31
|
|
Rosuvastatin Calcium
|
147098-20-2
|
USP/BP
|
|
Pitavastatin Calcium
|
147526-32-7
|
USP/BP
|
|
Fluvastatin
|
93957-54-1
|
USP31
|
|
Vinpocetine
|
42971-09-5
|
EP6.0
|
|
Atazanavir
|
198904-31-3
|
BP
|
|
Rosiglitazone
|
122320-73-4
|
USP30
|
|
Esomeprazole Magnesium
|
161973-10-0
|
USP/BP
|
|
Topiramate
|
97240-79-4
|
USP31
|
|
Fexofenadine HCl
|
153439-40-8
|
Inhouse
|
|
Bosentan
|
147536-97-8
|
Inhouse
|
|
D-Cysteine
|
921-01-7
|
Inhouse
|
|
D-Phenylalanine
|
673-06-3
|
Inhouse
|
|
Linagliptin
|
668270-12-0
|
Inhouse
|
|
Rivaroxaban
|
366789-02-8
|
USP
|
|
Saxagliptin
|
361442-04-8
|
USP
|
|
Vildagliptin
|
274901-16-5
|
USP
|
Major Pharmaceutical Intermediates
|
Items Descripation
|
Structure
|
Application
|
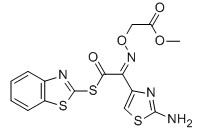
MICA ESTER
CAS No: 246035-38-1
Purity: ≥98%
|
|
For Cefixime
|
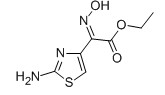
EHATA
CAS No: 64485-82-1
Purity: ≥98%
|
|
For Ceftazidine
|
2-Chloroadenine
CAS No: 1839-18-5
|
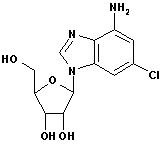
|
For Cladribine, Fludarabine et al
|
Bicyclo(2,2,1)Heptane-2,3-di-exo-carboximide
CAS No: 14805o-29-9
|
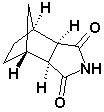
|
For Lurasidne
|
(R,R)-1,2-Bis(methanesulfonyloxy methyl)Cyclohexane
CAS No: 186204-35-3
|
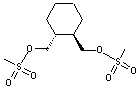
|
For Lurasidone
|
3-(Piperazin-1-yl)benzol[d] isothiazole
CAS No: 87691-87-0
|
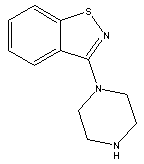
|
For Lurasidone
|
Trityl olmesartan
CAS No: 144690-92-6
Purity: ≥98%
|
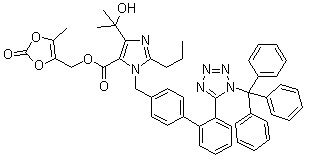
|
For olmesartan
|
3-Acetyl Pyridine
CAS No: 350-03-8
|
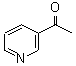
|
For Risedronate Sodium
|
3-(AceticAcid)pyridine HCL
CAS No: 6419-36-9
|
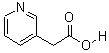
|
For Risedronate Sodium
|
Risedronic Acid
CAS No: 105462-24-6
|
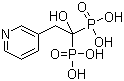
|
For Risedronate Sodium
|
3-Hydroxy-1-adamantyl-D-Glycine
CAS No: 709031-29-8
|
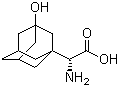
|
For Saxagliptin
|
(1s,3s,5s)-3-(aminocarbonyl)-2-azabicyclo(3,1,0) hexane-2-carboxylic acid tert-butyl ester
CAS No: 361440-67-7
|
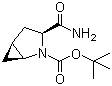
|
For Saxagliptin
|
(S)-N-Boc-3- hydroxy-adamantylglycine
CAS No: 361442-00-4
|
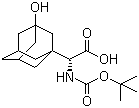
|
For Saxagliptin
|
2-Azabicyclo[3.1.0] hexane-3-carbonitrile, (1s,3s,5s)-
CAS No: 866083-42-3
|
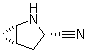
|
For Saxagliptin
|
Ethyl 3-(pyridin-2-ylamino) propanoate
CAS No: 103041-38-9
|
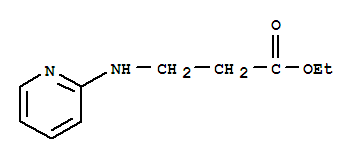
|
For Dabigatran
|
N-(4-Cyanophenyl) glycine
CAS No: 42288-26-6
|
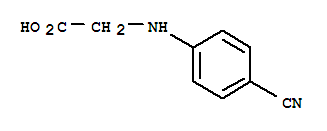
|
For Dabigatran
|
4-methylamino-3-nitrobenzoic Acid
CAS No: 41263-74-5
|
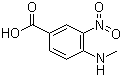
|
For Dabigatran
|
S-3-Amino-3-phenylpropanoic acid ethyl ester HCL
CAS No: 167834-24-4
|
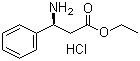
|
For Dapoxetine
|
(S)-3-Amino-3-Phemylpropan -1-ol
CAS No: 82769-76-4
|
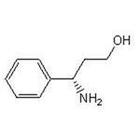
|
For Dapoxetine
|
(S)-3-Dimethylamino-3-Phemylpropanol
CAS No: 82769-75-3
|
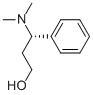
|
For Dapoxetine
|
4-{4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-butynil}-α,α-dimethyl benzene acetic acid
CAS No: 832088-68-3
|
|
For Fexofenadine HCl
|
Methyl 2-(4-(4-chlorobutanoyl)phenyl)-2-methylpropanoate
CAS No:154477-54-0
|
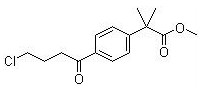
|
For Fexofenadine HCl
|
5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone
CAS No 461432-22-4
|
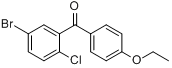
|
For Dapagliflozin
|
4-(5-Bromo-2-chlorobenzyl)phenyl ethyl ether
CAS No :461432-23-5
|
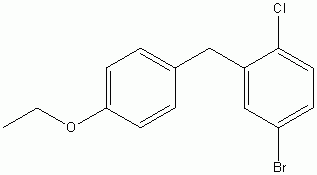
|
For Dapagliflozin
|
APIs & Intermediates
Mica Ester,Pharma Intermediates,Ciprofloxacin Hcl Uses,Active Pharmaceutical Ingredients
NINGBO VOICE BIOCHEMIC CO. LTD , https://www.pharma-voice.com